Vision
A Biotechnology Company to Improve Human Wellness
A Biotechnology Company to Improve Human Wellness
through Innovative Vaccines Development with
Recombinant Protein Platform Technology
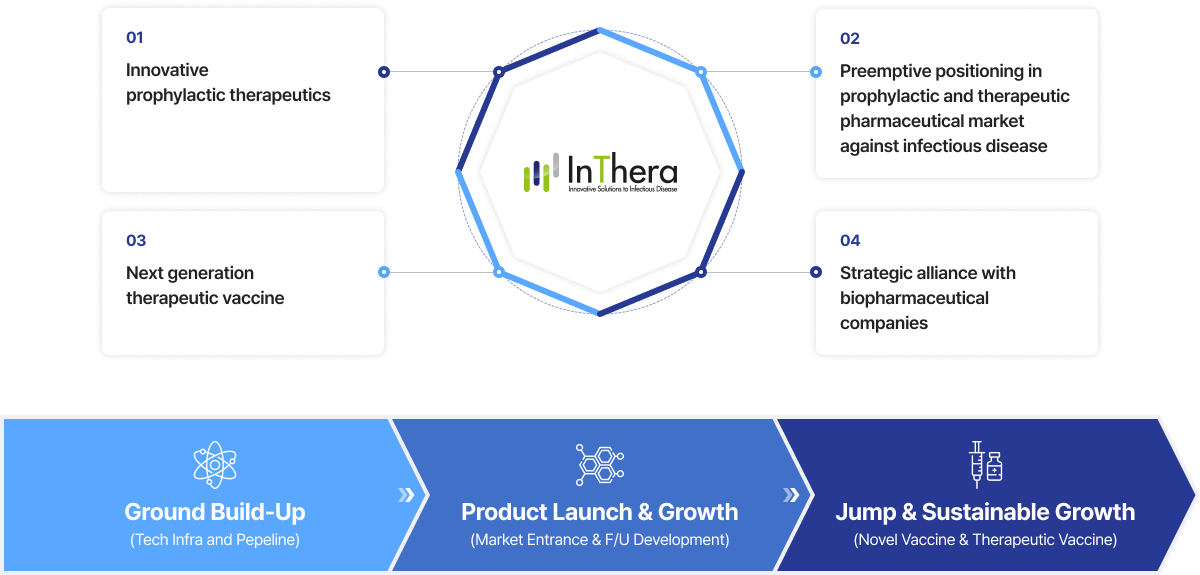
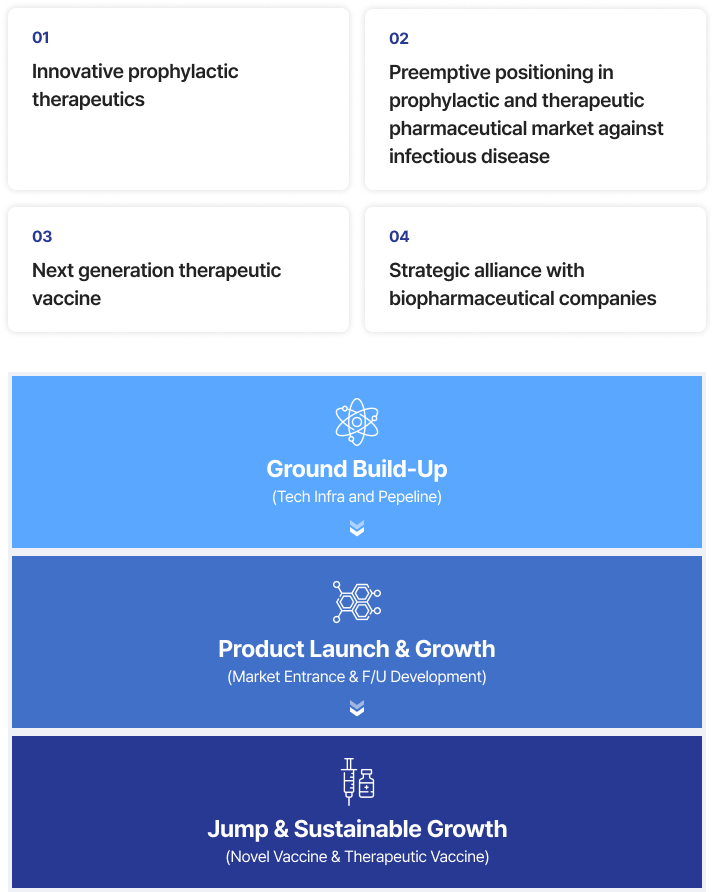
Through the development of innovative prophylactic & therapeutic
biopharmaceutical products, InThera aims to lead the technology
and products in the field of infectious disease market
InTherais exploring the innovative technology and novel products
in rapidly advancing infectious disease market
-
2022
- Bridge A Funding
- Preclinical studies for the Norovirus vaccine
- Preclinical Studies for the Rotavirus vaccine
-
2023
- IND application for Norovirus vaccine phase 1 trial
- Immunogenicity Evaluation for Zika/Dengue vaccines
-
2024
- Phase 1 trial for the Norovirus vaccine
- Protective Efficacy Evaluation for Rotavirus vaccine
- Manufacturing process optimization for Zika/Dengue vaccines
- Series B Funding
-
2025
- Out-Licensing for the Norovirus vaccine
- Phase 1 trial for the Rotavirus vaccine
- Out-Licensing for the Rotavirus vaccine
- Preclinical studies for Dengue vaccine
-
2026
- Phase 2 trial for the Rotavirus Vaccine
- IND approval for Norovirus vaccine phase 2 trial
- Phase 1 trial for Zika/Dengue Vaccines
- Pre-IPO Placement
Growth Momentum
-
Securing IP and Platform technology
- E. coli-based vaccine platform
- VLNP vaccine platform
- Expansion into cell-based vaccines
-
Establishing a Pipeline based on Platform technology
- Norovirus Vaccine
- Rotavirus Vaccine
- Next-generation Vaccines (Dengue/Zika)
-
Deriving Business Performance of New Vaccine Development
- WHO PQ (Pre-qualification)
- Supply of vaccine drug substances to LMICs (low- and middle-income countries)
- Out-licensing for main pipeline